Abstract
Nonmyeloablative (NMA) haploidentical T-cell replete HSCT is used increasingly due to the ability of alloreactive T-cells to enhance engraftment. Because T cells also can produce graft-versus-host disease (GVHD), post-transplant immunosuppressive therapy is required. GVHD also adversely affects thymic function and impairs immune reconstitution. Megadose T cell-depleted (TCD) haploidentical HSCT offers a low GVHD risk, even without post-transplant immune suppression, but achieving engraftment after NMA conditioning remains a barrier. Accordingly, we developed a strategy combining T cell-depleted megadose HSCT with veto cells, and demonstrated feasibility in preclinical models.
Veto activity is the ability of donor cells to attack host CTL-precursors targeting antigens expressed on the veto cells themselves, sparing other T cells, including anti-pathogen clones. Central memory (CM) CD8 T cells exhibit the most robust veto activity, but these cells may also produce GVHD. We separated the veto from GVH reactivity by expanding naïve or memory CD8 T cells against 3rd party MHC or viral antigens, respectively, under culture conditions favoring expression of a central memory phenotype; anti host clones are naturally depleted through death by neglect under cytokine deprivation at the early phase of the culture. Such anti-3rd party central memoryCD8 T cells enhance engraftment of T cell depleted bone marrow transplants without GVHD in fully mis-matched recipient mice (Reviewed in Reisner Y, Or-Geva N. Semin Hematol. 2019; 56 (3)).
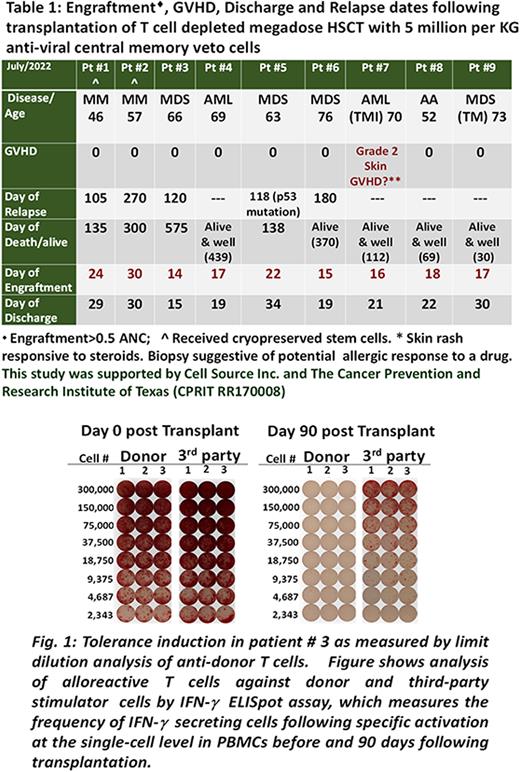
We initiated a phase I human clinical trial, to evaluate the safety and effectiveness of anti-viral CM veto cells to achieve engraftment without GVHD by day 42 post megadose TCD haploidentical HSCT using NMA conditioning. For production of the veto cells, donor memory CD45RO+ CD8 T cells are isolated and co-cultured with donor dendritic cells pulsed with peptides from four viruses (EBV, CMV, BKV, and Adenovirus). The culture is carried out under cytokine deprivation for 3 days to allow death by neglect of anti-host clones, then IL15, IL21 and IL7 are added to allow expansion of anti-viral central memory CD8 T cells. In 11 runs, >1x1010 CD45RO+CD3+CD8+CD62L+ T cells were generated from a single leukapheresis. At the end of 12 days of culture, there were 96.4± 2.5 % CD3+CD8+ T cells of which 80.1± 10.6 % (range 71.5 to 92.8) exhibited CD62L+CD45RO+ CM phenotype. Limiting dilution analysis revealed >3 log depletion of alloreactive T cell clones. Anti-viral activity tested by intracellular expression of TNF-α and INF-g showed an average of 38.8 ±19.6 % positive cells upon 6 hours stimulation against the viral peptide mixture. The study has enrolled 9 patients (Median age 66, range 46-76) (Table 1), 8 with hematologic malignancies and one with aplastic anemia. 7 patients received anti-thymocyte globulin 2 mg/kg daily on Day -8 to -6, fludarabine 30 mg/m2 on Day-5 to -2 and 3 Gy Total Body Irradiation (TBI) on Day-1. Two collections of donor G-CSF mobilized peripheral blood progenitor cells were performed on day 0 and day1. The first infusion was CD34 selected cells and the second was depleted of CD3+/CD19+ cells. The infusions included 1 to 2 x105 CD3+ T cells/kg. Cyclophosphamide (CY) 50 mg/kg was given on day 3 and 4. Veto cells (5x106 /kg) were infused on Day 7. Two patients (one with AML and one with MDS) were treated with the same regimen except for using total marrow irradiation, 12 Gy over 4 days instead of TBI. All patients engrafted. Apart from one patient who developed steroid-responsive, overall grade 2 GVHD of the skin, no additional patients developed GVHD. No toxicity attributable to the veto cells occurred. All patients had 100% donor myeloid chimerism and mixed T cell chimerism (43.1± 25.4%) by day 60. Elispot analysis revealed that host T cells exhibited specific tolerance towards donor HLA antigens (Fig.1). One patient with a prior kidney transplant from the same donor had immunosuppressive therapy stopped without kidney rejection. In conclusion, our data demonstrate reliable engraftment of haploidentical TCD HSCT combined with anti-viral CM veto CD8 T cells following a well-tolerated reduced intensity conditioning and show low rates of GVHD in the absence of immunosuppression beyond CY treatment on day 4. This approach deserves further study in allogeneic HSCT for malignant and nonmalignant hematologic diseases, as well as enhancement of tolerance for organ transplantation.
Disclosures
Champlin:General Oncology: Other: Data Safety Monitoring Board; Bluebird: Other: Data Safety Monitoring Board; Johnson &Johnson: Consultancy; Actinium: Consultancy; Omeros: Consultancy; Kadmon: Consultancy; Cell Source Inc.: Research Funding. Bachar Lustig:Cell Source Inc.: Patents & Royalties: Patent without Royalties at present. Oran:ASTEX: Research Funding; AROG: Research Funding. Alousi:Prolacta: Consultancy; Genetech: Consultancy; Mallinkrodt: Honoraria; Incyte: Honoraria, Research Funding; Sanofi / Kadmon: Honoraria. Popat:Novartis: Research Funding; Incyte: Research Funding; Bayer: Research Funding; Abbvie: Research Funding. Rezvani:Takeda: Patents & Royalties, Research Funding; GemoAb: Other: Participates on the Scientific Advisory Board ; AvengeBio: Other: Participates on the Scientific Advisory Board ; Virogin Biotech: Other: Participates on the Scientific Advisory Board ; GSK: Other: Participates on the Scientific Advisory Board ; Bayer: Other: Participates on the Scientific Advisory Board ; Navan Technologies: Other: Participates on the Scientific Advisory Board ; Caribou Biosciences: Other: Participates on the Scientific Advisory Board ; Affimed: Research Funding. Shpall:Takeda: Patents & Royalties; Bayer: Honoraria; adaptimmune: Consultancy; axio: Consultancy; Navan: Consultancy; Fibroblasts and FibroBiologics: Consultancy; NY blood center: Consultancy; Affimed: Other: License agreement. Reisner:Cell Source Inc.: Consultancy, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: Patent without Royalties at present, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.